Introduction
The Power and Complexity of Multispecific Antibodies
Multispecific antibodies (MsAbs) with multi-antigen binding capabilities have unique modes of action that set them apart from traditional monoclonal antibodies (mAbs). These complex molecules have garnered significant attention in recent years due to their versatility to target multiple pathways simultaneously. The growing approval of bispecific antibodies (BsAbs), particularly in oncology, demonstrates the value of this class of therapeutic proteins to address unmet medical needs.
MsAbs and BsAbs are notoriously difficult to express in the correct format due to challenges with dimerization of antibody halves, chain mismatches, and aggregation during expression. As a result, production often requires extensive optimization in both expression and purification processes.
Traditional mammalian cell-based expression systems, like CHO cells, have limitations in producing MsAbs due to cost and time constraints. These systems require the transfection of cells with two different heavy chains and one or two light chains to promote correct assembly. The process is highly complex, often requiring specialized purification methods and careful optimization of transfection ratios to achieve the necessary yield and purity.
Despite these challenges, the use of MsAbs in therapeutic development continues to expand, driven by their potential to offer more targeted, effective treatments for a range of diseases.
The Protein in Profile: Runimotamab – A Dual-Targeting Antibody for Cancer Immunotherapy
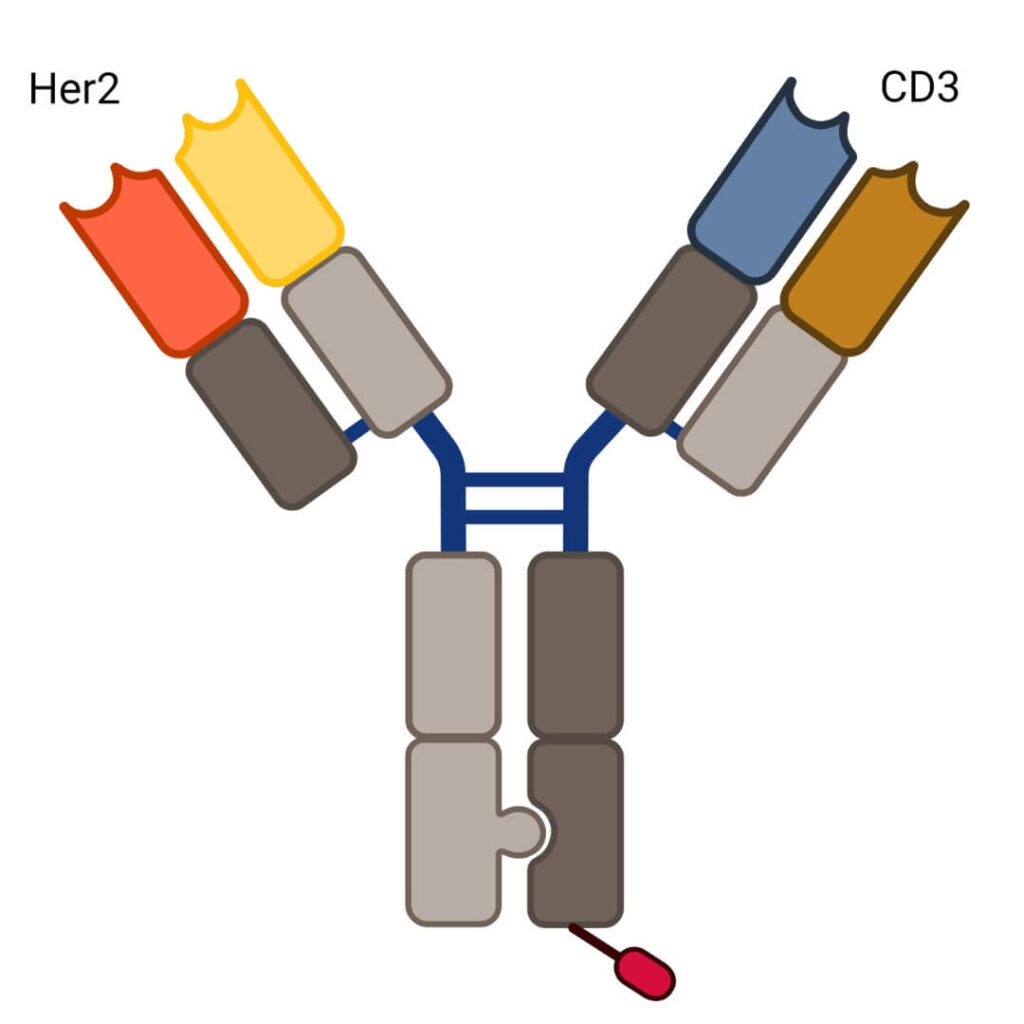
Runimotamab is an anti-HER2-anti-CD3 BsAb currently in clinical development by Genentech. Runimotamab targets both HER2-positive tumor cells and CD3 on T-cells, inducing a polyclonal T-cell response to attack HER2-positive tumors.
The BsAb was designed using the Knobs-Into-Holes (KIH) technology, a strategy used to enable correct heavy chain pairing of BsAbs. KIH involves engineering specific amino acid mutations in the Fc region to promote accurate heterodimerization, improving the specificity and stabilizing the structure during production.
Another key feature of Runimotamab is the lack of glycosylation in its Fc region, engineered to silence the antibody’s Fc functionality. The N-glycosylation motif has been silenced by modifying the asparagine residue to glycine, preventing glycosylation from occurring.
Runimotamab was produced in CHO cells and human expression systems. Despite the use of KIH, cell-based expression challenges remain, particularly in ensuring correct assembly and maximizing yields, both of which are critical for its clinical application.
Streamlining Production Through Cell-Free Expression
The expression of BsAbs like Runimotamab presents several technical challenges which can result in low yields, mispairing of chains, and difficulties in ensuring proper post-translational modifications (PTMs).
Chain mispairing: Runimotamab’s complex structure, which involves the assembly of two distinct heavy and two distinct light chains, increases the risk of chain mispairing. Correct assembly is crucial for the antibody’s functionality, and even minor mispairing can lead to reduced therapeutic efficacy.
Cell-based expression challenges: Expression of BsAbs in CHO cells is often associated with longer timelines, high costs, and risks of contamination. In traditional mammalian cell systems, optimizing conditions for BsAb production—such as transfection ratios and ensuring correct PTM processes—can further increase time, costs and effort.
Given these challenges, cell-free expression systems offer a flexible and scalable alternative for producing Runimotamab. The ease of use and short experimental iteration times inherent to cell-free systems provide the agility to easily and rapidly optimize production conditions, such as DNA template concentration and additives, including chaperones and reducing/oxidizing agents. By eliminating the need for cell line development and maintenance, cell-free expression bypasses the limitations of CHO-based production, enabling rapid protein synthesis within the same system throughout development. This not only reduces the risk of contamination but also significantly accelerates the development timelines of complex protein classes like MsAbs and BsAbs.
Materials and Methods
Optimizing Expression with the ALiCE® Cell-Free Platform
The ALiCE constructs were prepared and the expression reaction set up with the following parameters:
- As disulfide bonds are required for the assembly of full-sized antibodies, the heavy and light chains of Runimotamab were cloned into the pALiCE02 vector, which targets the protein to the microsome, a vesicular structure where disulfide bonding takes place.
- A strep-tag was included in the construct to enable an additional round of purification if required.
- Various plasmid concentrations and construct ratios (heavy to light chain) were tested to optimize expression.
- Two different expression strategies, using either two or four plasmids, were compared. The 4-plasmid method uses separate plasmids for each subunit: HER2 light chain, HER2 heavy chain, CD3 light chain, and CD3 heavy chain. The 2-plasmid method combines the light and heavy chains of HER2 on one plasmid (HER2 duet) and the light and heavy chains of CD3 on another plasmid (CD3 duet).
- 50 μL ALiCE® reactions were set up according to the user manual guidelines, and the expression reaction ran for 24 and 48 hours.
Results
Expression Achieved Under All Conditions Tested
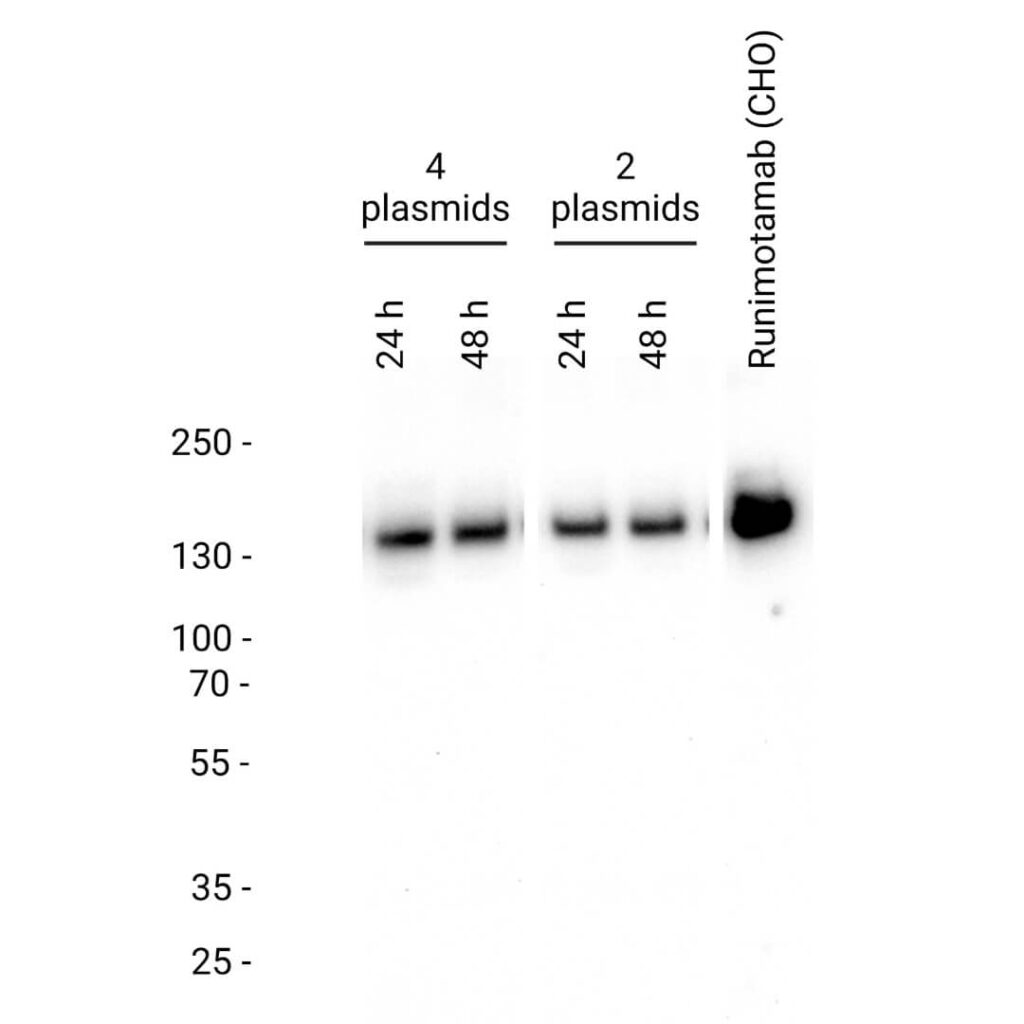
- Runimotamab was successfully expressed at both 24 and 48 hours, with detection performed using an anti-human IgG antibody.
- Both 4-plasmid and 2-plasmid co-expression strategies achieved complete assembly of Runimotamab within 24 hours, with protein size comparable to CHO-produced antibodies with western blot analysis
- Since the 2-plasmid approach yielded similar expression levels to the 4-plasmid method, it was chosen for all subsequent expressions due to its efficiency and lower cost of goods (COGs).
KEY OPTIMIZATION STRATEGY
The initial assumption that a 1:1 ratio of the Knob to Hole plasmid DNA would be optimal for this protein’s expression proved to be incorrect. After testing various ratios, a 1:2 ratio was identified as the most effective, with a final plasmid concentration set at 15 nM.
Full Assembly and Integrity Retained Post-Purification
- Runimotamab was purified using one-step Protein A chromatography from DDM-disrupted microsome fractions as described in the ALiCE® user manual. The eluted protein was neutralized and concentrated.
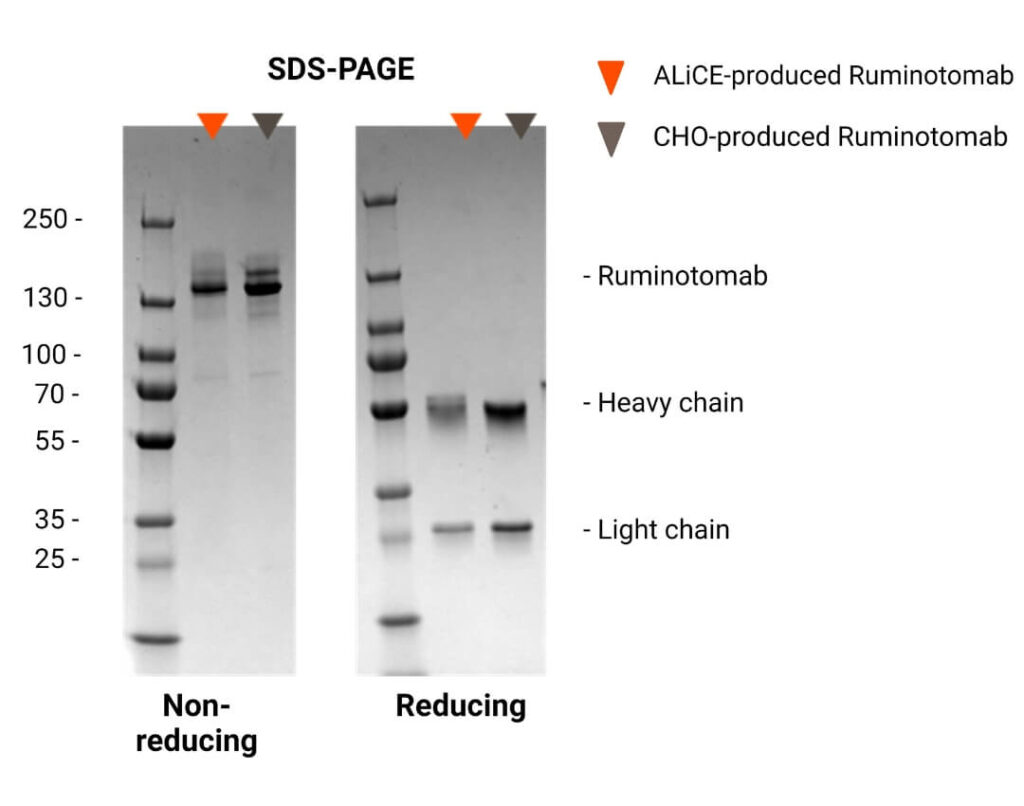
- Under non-reducing conditions, a single band corresponding to the CHO-produced antibody was observed.
- Under reducing conditions, separate bands for the heavy and light chains were detected, confirming the correct assembly and integrity of the expressed BsAb.
KEY OPTIMIZATION STRATEGY
During Protein A purification, a pH 9 neutralization buffer was added to the elution tubes prior to elution. This step is crucial for maintaining protein stability, as Protein A elutions typically occur at a low pH of 2.5–3.5, which can destabilize the antibody. Adding the neutralization buffer ensured the eluted antibody was maintained at a near-neutral pH, preserving its structural integrity and functionality.
High Yield and Purity
- After a single round of Protein A purification, Runimotamab expressed using ALiCE® achieved a yield of 50 mg/L with a purity of 83%.
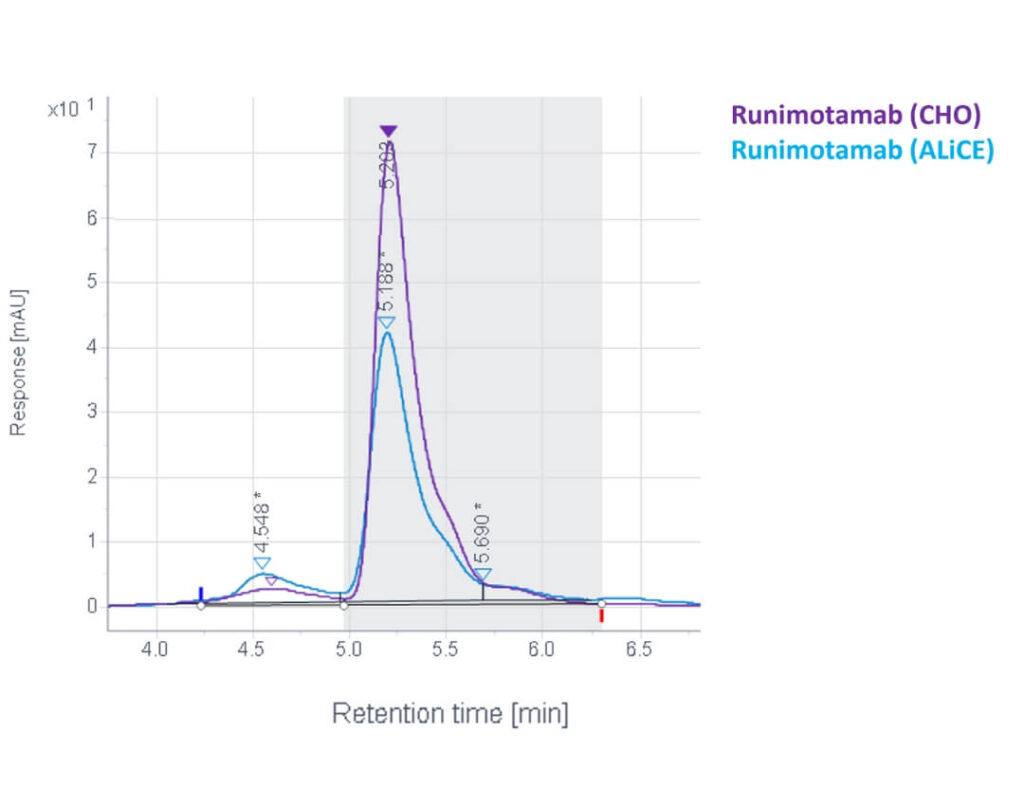
- Both ALiCE®– and CHO-derived Runimotamab were similar in terms of purity and profile, with minor differences observed in impurity levels.
- ALiCE®-expressed Runimotamab showed slightly higher molecular weight species, which could be further removed with additional purification steps.
KEY OPTIMIZATION STRATEGY
To improve purity, a MabSelect PrismA column – highly specific for antibody purification – was used during downstream processing. This ensured the effective capture of the antibody while maintaining its integrity.
High Stability with No Propensity for Aggregation
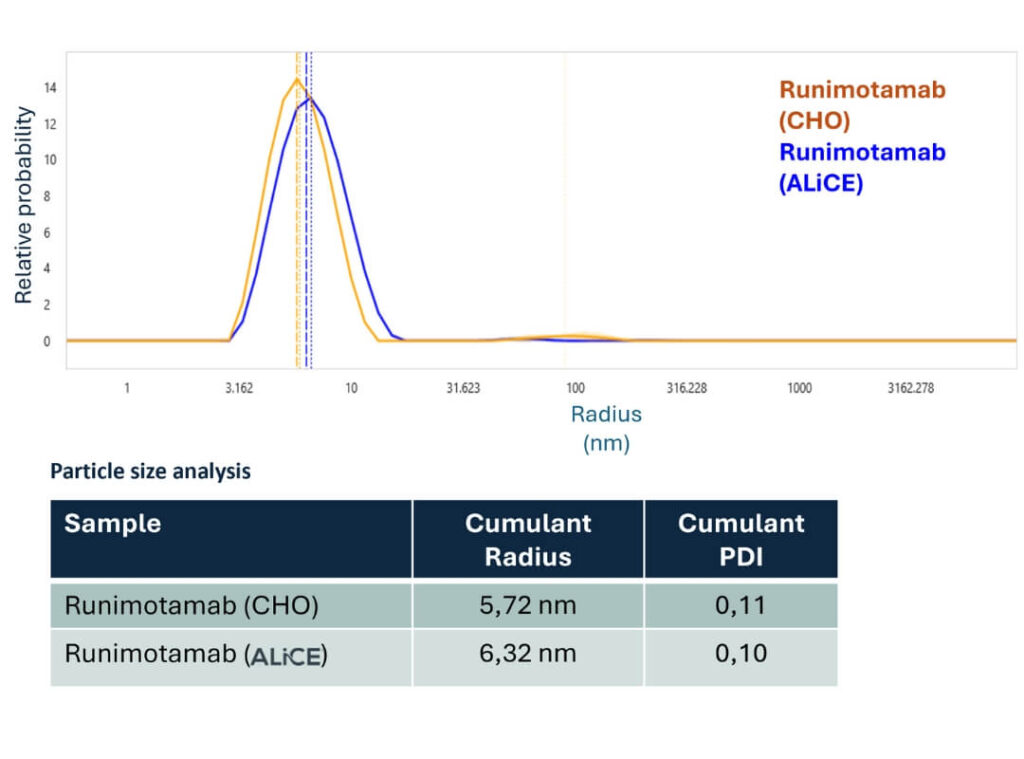
- Dynamic light scattering (DLS) analysis showed that both CHO- and ALiCE®-derived Runimotamab samples had low polydispersity index (PDI) values (<0.20), indicating monodisperse, stable samples.
- The slight size difference between CHO (5.72 nm) and ALiCE® (6.32 nm) antibodies may reflect subtle structural differences between expressions in each platform.
- No aggregation was observed in either product, as shown by their similar colloidal properties.
Comparable Target Binding Affinity
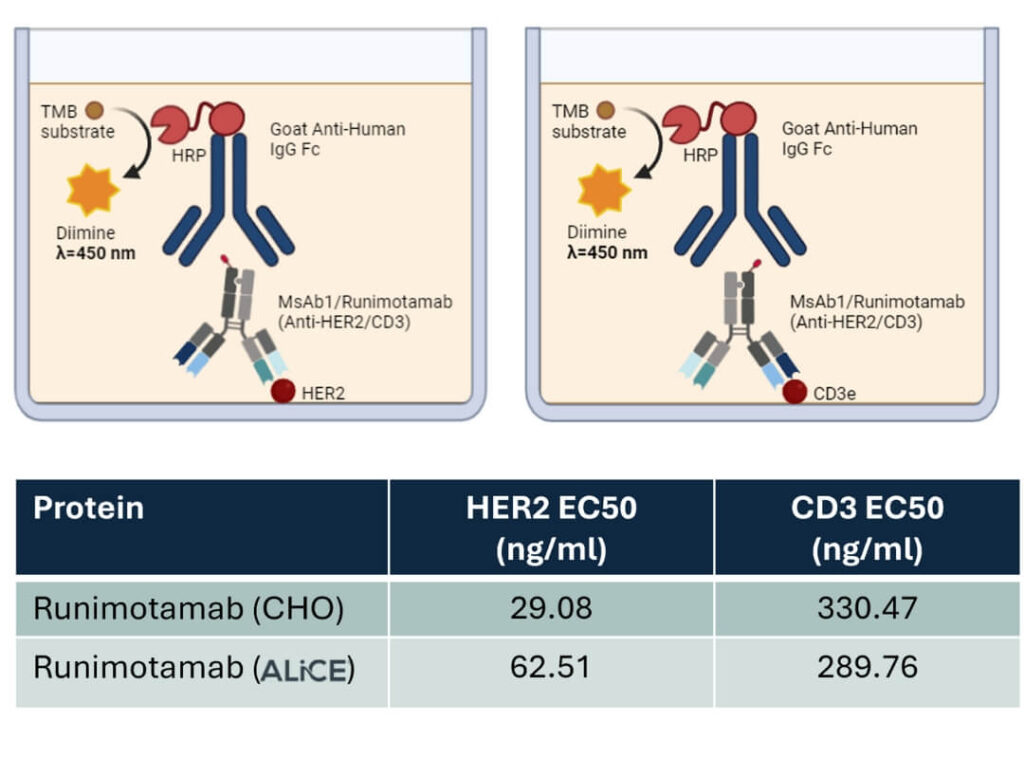
- A direct ELISA assay was conducted to evaluate the binding capabilities of ALiCE® – and CHO-derived Runimotamab to its two target antigens, HER2 and CD3. Commercially purchased HER2 and CD3 proteins were plated, and various dilutions of purified Runimotamab were tested for binding efficiency.
- Both ALiCE®– and CHO-derived Runimotamab bound to their target antigens in a dose-dependent manner, indicating functional target engagement.
- The binding EC50 values revealed that ALiCE®-derived Runimotamab had 2.1 times weaker binding affinity to HER2 and 1.14 times stronger binding affinity to CD3 compared to the CHO-derived Runimotamab, suggesting minimal impact of the expression system on overall binding capabilities.
- The observed differences in binding will be further validated by quantifying the percentage of correctly formed heterodimeric BsAbs present in each sample to better correlate binding efficiency with protein structure.
Key Benefits of ALiCE®
Faster, Flexible, and Streamlined Production of a BsAb
The ALiCE® cell-free system enabled faster expression and provided the flexibility to optimize DNA template ratios, which are critical factors in expressing complex BsAbs like Runimotamab. This level of control over expression conditions would be challenging to achieve with traditional mammalian systems, making ALiCE® a valuable alternative for streamlining production. This proof-of-concept experiment demonstrates that ALiCE® can achieve comparable results but in a much faster and more efficient manner.
Successful Expression of a Knob-in-Hole BsAb in ALiCE®
The successful expression of Runimotamab in ALiCE® validates its potential for producing structurally complex antibody formats. Adjusting the ratio of Knob to Hole plasmid DNA led to more than 95% correct Knob-Hole heavy chain pairing, leading to improved yield and quality of the expressed BsAb.
Single-Plasmid Co-Expression for Reduced COGS
The heavy and light chains of each half of the antibody were cloned onto the same plasmid, allowing the production of two proteins from a single plasmid. This approach streamlined the expression process and significantly reduced the COGs.
ALiCE® for Other Complex Protein Expression
The in-house development of an ELISA assay for the two target antigens not only facilitates the evaluation of Runimotamab but also paves the way for establishing similar assays for other BsAbs and MsAbs. Functional assays, such as T-cell cytotoxicity to confirm the therapeutic activity, and surface plasmon resonance (SPR) studies to further characterize binding affinities and kinetics, will be performed.
The success of expressing Runimotamab using the ALiCE® platform confirms its potential as a versatile and scalable solution for producing complex protein formats that are challenging to express with traditional methods. This achievement sets the stage for exploring additional formats, such as CrossMab and common light chain approaches.
Developing a bispecific?
Connect to discuss your needs:
References
- Surowka, M., & Klein, C. (2024). A pivotal decade for bispecific antibodies?. mAbs, 16(1), 2321635. https://doi.org/10.1080/19420862.2024.2321635
- Klein, C., Brinkmann, U., Reichert, J. M., & Kontermann, R. E. (2024). The present and future of bispecific antibodies for cancer therapy. Nature reviews. Drug discovery, 23(4), 301–319. https://doi.org/10.1038/s41573-024-00896-6
- A study of Runimotamab in participants with locally advanced or metastatic HER2-expressing cancers. https://clinicaltrials.gov/study/NCT03448042
- Staflin, K. et al. (2020). Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI insight, 5(7), e133757. https://doi.org/10.1172/jci.insight.133757
- Li, J. et al. (2018). IFNγ-induced Chemokines Are Required for CXCR3-mediated T-Cell Recruitment and Antitumor Efficacy of Anti-HER2/CD3 Bispecific Antibody. Clinical cancer research : an official journal of the American Association for Cancer Research, 24(24), 6447–6458. https://doi.org/10.1158/1078-0432.CCR-18-1139
- Ong, H. K., Nguyen, N. T. B., Bi, J., & Yang, Y. (2022). Vector design for enhancing expression level and assembly of knob-into-hole based FabscFv-Fc bispecific antibodies in CHO cells. Antibody therapeutics, 5(4), 288–300. https://doi.org/10.1093/abt/tbac025
