Low protein yields are a common problem in many expression experiments, regardless of the type of protein – antibodies, enzymes, antigens, etc. – you are looking to produce. When protein expression falls below a certain concentration threshold, protein purification becomes difficult if not impossible. We touch on the potential causes of low yields in our article “Maximize Protein Expression: Overcoming Challenges and Boosting Yields“.
Here, we deep dive into the impact of protein solubility on protein yield, and how to enhance the solubility of your protein of interest. Specifically, we look at the role of solubility tags (also known as stability tags) and show how combined with a cell-free expression system like ALiCE®, it is possible to rapidly test different constructs and tags for optimal expression.
Why is protein solubility important?
The solubility of a protein can have a profound effect on protein functionality, purification efficiency, protein stability, and ultimately protein yield:
Protein functionality: If a protein has poor solubility, it may aggregate or form inclusion bodies of misfolded functionally-impaired protein. Hence, when a protein is insoluble, the yield of functional protein decreases.
Purification efficiency: Insoluble proteins require additional steps to solubilize and refold them into their native conformation, often involving denaturation and subsequent refolding. These are challenging addition steps and lead to further losses in protein yield.
Protein instability: An insoluble protein can be more prone to degradation or proteolysis during purification, leading to further losses in protein yield. Therefore, by addressing protein insolubility, it is possible to significantly improve overall protein yields.
Solubility tagging as a solution to protein insolubility
Solubility-enhancing tags are proteins or peptides that are fused to a target protein to improve its solubility and expression in heterologous expression systems.
These tags tend to be highly expressed and very soluble. Fusion of the tag to the N- or C- terminus of your target protein, can thus enhance solubility and expression of the passenger protein. Tags may also provide a chaperone function, assisting in correct protein folding.
In our protein service, our team of in-house expression experts test various tags to optimize protein solubility for our clients, including the Maltose Binding Protein (MBP) tag and the SUMOstar tag. The simplicity and speed of our ALiCE® expression system, which can produce proteins within 24 hours of DNA addition, allow them to test various expression constructs in parallel to determine optimum protein production conditions.
The MBP tag in action – a case study
Our protein service team was tasked with expressing a viral coat protein with known protein solubility issues. As the protein contains disulfide bonds, the team used the pALiCE02 vector backbone which targets the protein to the microsomes for post-translational processing via the Melitin Signal Peptide (MSP). Read more about the role of microsomes in ALiCE® here.
Two versions of the construct were generated (figure 1a). Construct 1 contained the gene-of-interest cloned into a standard pALiCE02 plasmid containing a C-terminal Strep-tag. Construct 2 contained an additional C-terminal MBP tag. Plasmids were added to the ALiCE lysate in concentrations of 5, 7.5 and 10 nm and the expression reaction run according to our standard protocol. Protein expression was analyzed after 24 hours incubation.
The lysate was processed to release the proteins from the microsomes (see our protocol for details). Anti-Strep Western blot analysis, showed only a very faint product at 10 nm for construct 1 (figure 1b), while construct 2, containing the MBP tag, produced strong protein product bands at all plasmid concentrations (figure 1c).
Using the ALiCE® system for expression, allowed the team to test these different constructs rapidly without lengthy strain creation or optimizations of the reaction mix.
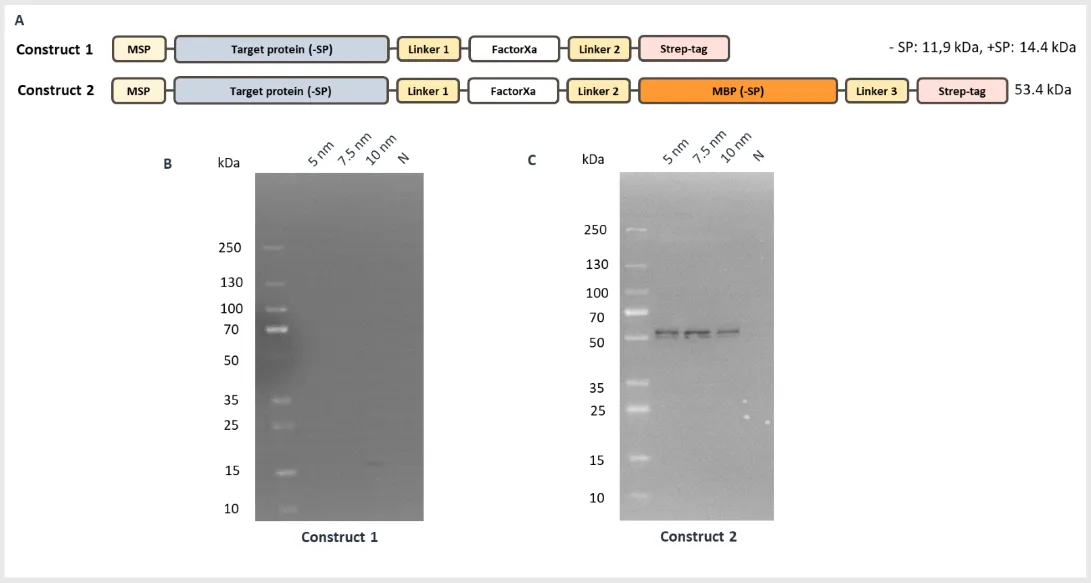
Figure 1. Viral coat protein expression with and without the MBP tag. (A) Design of the two constructs (-/+ MBP); (B) Anti-Strep Western blot of MBP- construct 1 with a faint protein product band at 10 nm plasmid concentration; (C) Anti-Strep Western blot or MBP+ construct with strong bands at all plasmid concentrations.
Conclusion
While there is no one-fits-all solution for increasing protein solubility, our data shows that in our hands, solubility tagging can significantly improve both solubility and yields of certain proteins.
Furthermore, should this not be the right solution for your protein, our protein service team are ready to develop a specific expression and solubilization strategy for your specific protein needs.
